When a generic drug company submits an application to the FDA, not all applications are treated the same. Some get fast-tracked. Others wait. The difference? Priority review versus standard review. This isn’t about favoritism - it’s about public health needs, market gaps, and supply chain security. Understanding how the FDA decides which generic drugs get reviewed faster can help explain why some life-saving medications hit shelves months before others.
What Are Priority and Standard Review?
The U.S. Food and Drug Administration (FDA) uses two main review tracks for generic drug applications, known as Abbreviated New Drug Applications (ANDAs). These tracks set clear deadlines for decision-making. Standard review gives the FDA 10 months to complete its evaluation. Priority review cuts that time to 8 months. That 2-month difference might seem small, but in the world of generic drugs, it can mean millions in revenue - or a life-saving drug reaching patients sooner.
These timelines come from the Generic Drug User Fee Amendments (GDUFA) III, which took effect in October 2022 and runs through 2027. Before GDUFA, review times were less predictable. Now, companies know exactly what to expect - if they qualify.
Standard review applies to most ANDAs. It’s the default path. But if your drug meets one of the FDA’s specific criteria, you jump to priority review. The key triggers? First generics, drugs in shortage, or those that offer a meaningful clinical advantage over existing options.
Who Gets Priority Review?
Not every generic drug company can request priority review. The FDA sets strict rules. Only three types of applications qualify:
- First generics - The first company to submit a complete ANDA for a brand-name drug after its patents and exclusivity expire. This is the most common reason for priority review. In 2022, over 90% of first generics received 180 days of market exclusivity, giving them a huge financial edge.
- Drugs in shortage - If the FDA determines a drug is in critical shortage (as defined under Section 506C of the Federal Food, Drug, and Cosmetic Act), its generic version gets priority. This matters most for older, low-cost medicines like antibiotics or chemotherapy drugs where supply disruptions can be deadly.
- Medically important advances - Rare, but powerful. If a generic version offers better safety, dosing, or stability than existing versions - even if another generic already exists - it may qualify. This is often used for complex products like inhalers or injectables with improved delivery systems.
The FDA’s Orange Book database tracks patent and exclusivity status, so companies know exactly when they can file. Missing the window by even a day can cost you the priority slot - and the financial upside.

The New Domestic Manufacturing Pilot
In October 2023, the FDA launched a game-changing addition: the ANDA Prioritization Pilot Program. This isn’t just about speed - it’s about security. The program gives priority review to ANDAs that meet three strict U.S.-based criteria:
- The bioequivalence studies were conducted in the United States.
- The finished dosage form (the pill or injection you take) is manufactured in the U.S.
- The active pharmaceutical ingredient (API) comes entirely from U.S. suppliers.
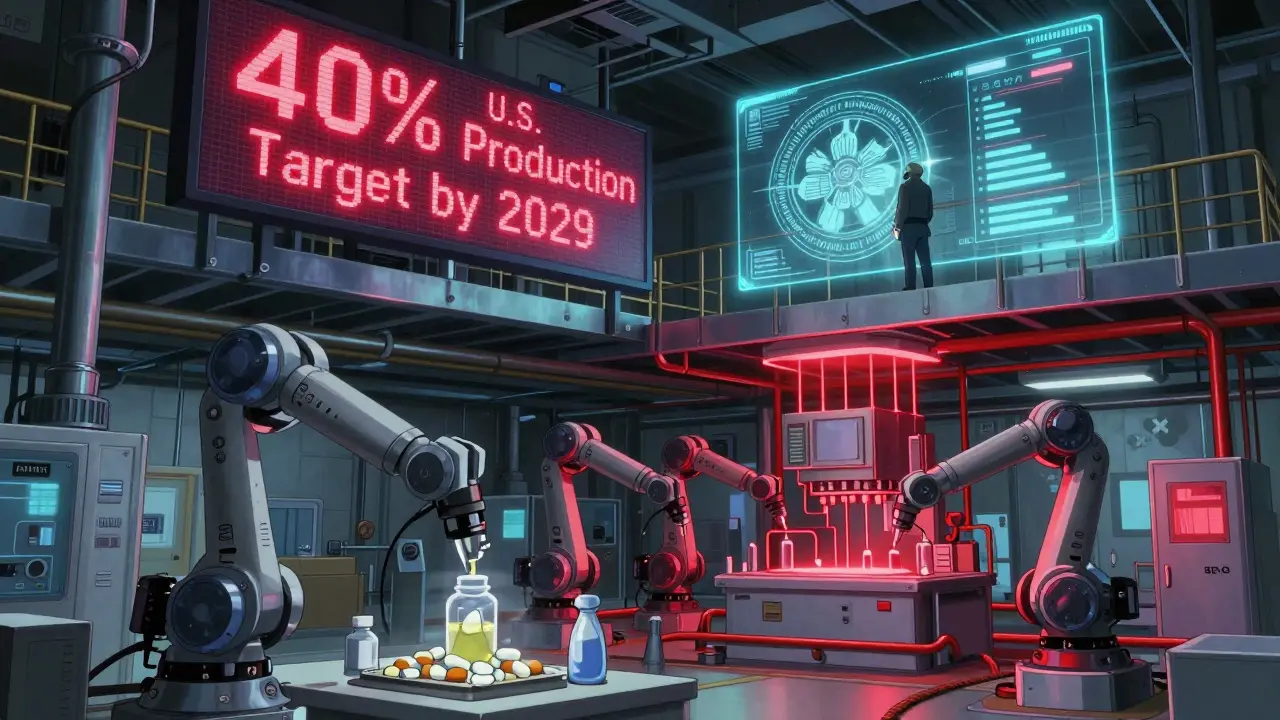
This initiative directly responds to the fragility exposed during the pandemic. In 2021, the FDA reported that 80% of APIs - the core chemical ingredients in drugs - were made overseas. When global supply chains broke down, shortages spiked. Now, the FDA is incentivizing U.S. production.
But here’s the catch: only 12.3% of generic drug sponsors currently meet all three requirements, according to FDA facility data. That means most companies will need to rebuild their supply chains to qualify. Some are already doing it. Teva, Sandoz, and Hikma have increased U.S. bioequivalence testing by over 20% since the pilot launched. Contract research firms like Covance report a 35% surge in U.S.-based studies.
For companies that make the switch, the payoff is real. One manufacturer told industry analysts their cardiovascular drug could generate $120 million more in revenue by entering the market two months earlier. That’s the power of priority review.
Why the Review Process Often Gets Delayed
Even if you qualify for priority review, that doesn’t mean your application clears the FDA quickly. The biggest roadblock? Incomplete or poorly prepared submissions.
Every ANDA goes through a 74-day filing review. If it’s missing key data - say, incomplete chemistry reports or unclear bioequivalence data - the FDA issues a Refuse-to-Receive (RTR) letter. You lose your place in line. You pay another $164,880 filing fee. And you start over.
Even after filing, problems arise. In 2022, 31.7% of ANDAs received at least one Complete Response Letter (CRL), meaning the FDA asked for more data. Of those, nearly half (47.2%) were due to Chemistry, Manufacturing, and Controls (CMC) issues. These aren’t minor fixes. They’re often deep problems with how the drug is made - impurity levels, stability testing, or packaging compatibility.
The average ANDA needs 1.7 review cycles to get approved. Each cycle adds about 4.2 months to the timeline. That’s why many generics still take over two years to reach market after patent expiry.
How Companies Are Adapting
To beat the system, smart companies are changing how they work. Pre-submission meetings with the FDA - once rare - are now common. In 2020, only 41% of sponsors held these meetings. By 2023, that number jumped to 63%. These early discussions help avoid costly mistakes.
The FDA also launched the Complex Generic Drug Product Pilot Program in January 2023. It targets hard-to-make products like topical creams, inhalers, and extended-release tablets. These account for 18.3% of pending applications but only 9.7% of approvals. With early guidance, approval rates for these complex drugs are improving.
And the future? AI is coming. The FDA is testing automated tools to screen straightforward ANDAs. In internal trials, these tools cut review times by 18.7%. By late 2024, they’ll be rolled out more widely. That could mean even faster approvals - especially for simple, low-risk generics.
The Bigger Picture: Why This Matters
Generic drugs make up 88.6% of all prescriptions in the U.S., but only 15.3% of drug spending. That’s how the system saves money - by letting cheaper alternatives compete after patents expire. But if the FDA can’t approve those generics fast enough, patients pay more.
The average time from patent expiry to first generic approval is still 2.7 years. That’s too long. The new pilot programs, faster reviews, and U.S. manufacturing push are all aimed at cutting that delay. The FDA aims to increase U.S.-made generic drugs from 28% to 40% within five years. That’s not just about jobs - it’s about resilience.
When a shortage hits, and a hospital runs out of a critical drug, the difference between a 10-month and 8-month review can be the difference between life and death. The FDA’s system isn’t perfect - but it’s evolving. And for the companies that understand how to play by its rules, the rewards are huge.
What’s the difference between priority review and standard review for generic drugs?
Standard review takes 10 months from submission date, while priority review takes 8 months. Priority review is only given to applications that meet specific criteria: first generics, drugs in shortage, or those offering a meaningful clinical improvement. The faster timeline helps get critical medications to patients sooner.
Can any generic drug company apply for priority review?
No. Only applications that meet one of three criteria qualify: (1) being the first generic to market after a brand-name drug’s exclusivity expires, (2) addressing a drug shortage recognized by the FDA, or (3) offering a significant clinical advantage over existing generics. The FDA evaluates each application against strict guidelines.
What is the ANDA Prioritization Pilot Program?
Launched in October 2023, this program gives priority review to generic drug applications that use U.S.-based bioequivalence testing, U.S. manufacturing of the finished product, and U.S.-sourced active pharmaceutical ingredients (APIs). It’s designed to strengthen domestic supply chains and reduce reliance on foreign manufacturing.
Why do so many generic drug applications get rejected or delayed?
Most delays come from incomplete or poorly prepared submissions, especially in Chemistry, Manufacturing, and Controls (CMC). In 2022, nearly half of all Complete Response Letters (CRLs) were due to CMC issues. Poorly documented stability data, impurity profiles, or manufacturing processes often trigger these delays, requiring companies to resubmit with more data - and lose valuable time.
How has the FDA improved the approval process for complex generics?
The FDA launched the Complex Generic Drug Product Pilot Program in January 2023 to provide early scientific advice for hard-to-develop products like inhalers, topical creams, and extended-release tablets. Companies that participate in this program have seen their first-cycle approval rates jump from 24.1% to 38.7%, significantly reducing delays.
Are AI tools being used to speed up generic drug reviews?
Yes. The FDA is testing AI-assisted tools to screen straightforward ANDAs. In internal trials, these tools reduced review times by 18.7%. A full rollout is expected by Q3 2024, which could accelerate approvals for simple generics and free up FDA staff to focus on more complex applications.